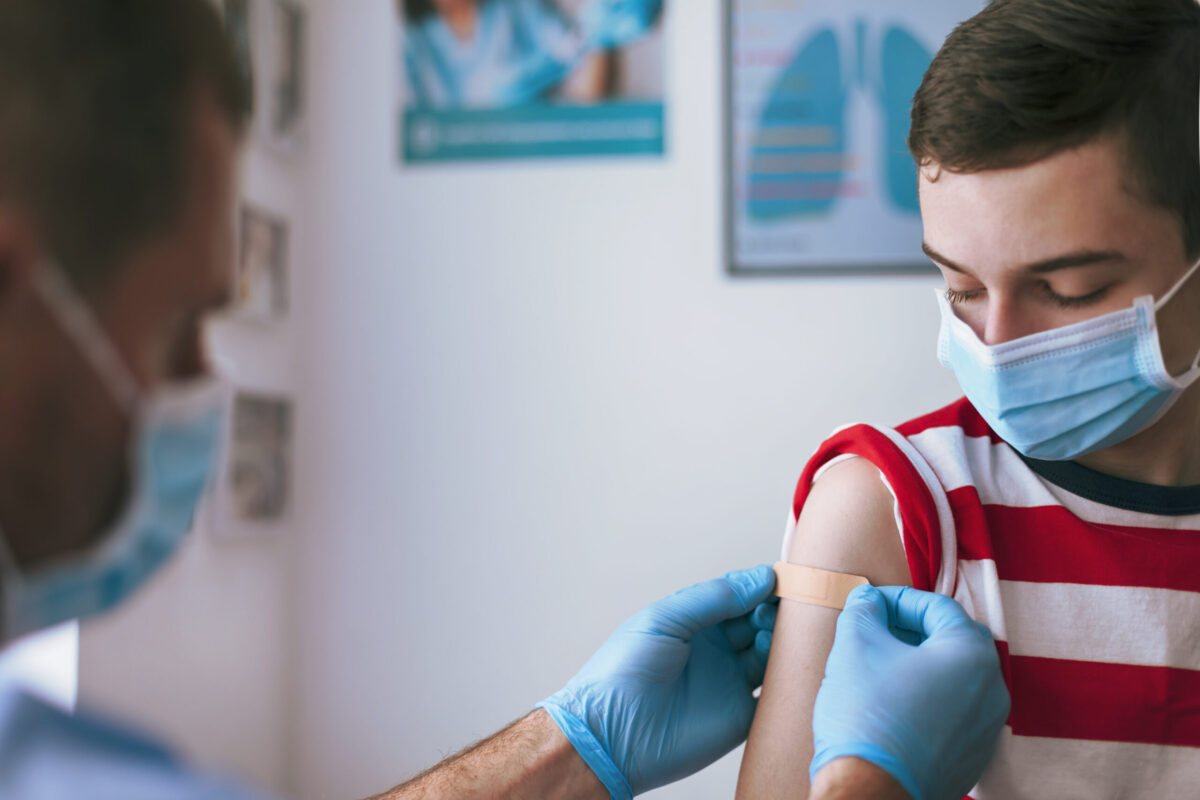
On May 11, Pfizer received Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA) for its COVID-19 vaccine for children ages 12-15. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices’ endorsement of its safety and effectiveness followed on May 13.
As of May 14, local health departments in Tennessee can vaccinate these children.
While Pfizer is the manufacturer of but one of the three widely available COVID-19 vaccines, the news of its vaccine’s authorization came as a relief for many preteens and teenagers waiting their turn — and an even greater relief for many parents and caregivers.
As you weigh whether to have your adolescent vaccinated, consider these four factors.
1. EUA means this vaccine has been found to be safe and effective for adolescents.
EUA is used to help make certain medical products available quickly during a declared health emergency like the COVID-19 pandemic.
Once an EUA request is submitted to the FDA, they evaluate and determine whether the relevant safety and efficacy criteria are met and if there’s a substantial public health benefit. During this process, they take into account all available scientific evidence and clinical trial results.
Currently, all available vaccines for adults and children alike are being administered under EUA.
2. COVID-19 infection remains a danger for adolescents.
While the majority of COVID-19 cases in children are mild, particularly in young children, it’s important for parents and caregivers to understand that children continue to be infected.
Between March 1 and December 12 of last year, more than 2.8 million laboratory-confirmed cases of COVID-19 in children, adolescents, and young adults age 0–24 years were reported in the U.S. While the majority of these cases (more than 57%) occurred among young adults between 18 and 24, adolescents aged 14–17 accounted for 16.3% of cases, and 11-to-13-year-olds accounted for nearly 8%.
In addition, Black and Latino children are more likely to be affected by health disparities and are disproportionately vulnerable to severe COVID-19 complications.
3. Clinical trial results were overwhelmingly positive.
Pfizer earned EUA for its vaccine after sharing data showing that 2,260 participants age 12-15 participated in a randomized, placebo-controlled clinical trial. There, 1,131 adolescents received the vaccine, and 1,129 received a saline placebo.
More than half of the participants were monitored for safety for at least two months following the second dose. Commonly reported side effects were pain at the injection site, tiredness, headache, chills, muscle pain, fever and joint pain, typically lasting 1-3 days. The side effects in adolescents were consistent with those reported in clinical trial participants age 16 and older.
With the exception of pain at the injection site, more adolescents reported these side effects after the second dose. Parents are advised to let their children know to expect some side effects after either dose, but more so after the second.
4. Parents can take steps now to have their adolescents vaccinated.
Vaccination appointments can be made at vaccinate.tn.gov. When making an appointment online, parents should select a Pfizer vaccine appointment time. Most health departments also accept walk-ins.
Before the appointment, be direct with your child about the pros and cons of vaccination. Ask open-ended questions to help you understand any worries your child may have way. For example, “What have you heard from friends or from the news about this vaccine?”
Research ahead of time to alleviate any concerns they may have about reported short-term effects of vaccination compared to confirmed long-term effects of COVID-19. It may help to remind kids that we already get vaccinated against the flu every year.
If they’re concerned about why younger relatives and friends can’t get the vaccine, discuss how clinical trials for younger children continue, and why younger developing immune systems have to be monitored longer.
Most importantly, stress the safety and the trust you have in medical experts. Consider giving your child some power of their own, such as continuing to wear a mask even after full vaccination based on their comfort level, following the CDC’s recent guidance that fully vaccinated people no longer need to wear masks indoors or outdoors.
Need more advice?
If you have questions or concerns about vaccines based on your health status, speak to a provider who knows your medical history. Your friends and family may have good intentions, but they may not know your body like you and your doctor, so it’s important to speak to a provider who knows you well.
If you do decide to go online to learn more about vaccines, do seek reputable sources like the CDC, FDA or World Health Organization (WHO). You can also visit BCBSTupdates.com to get the latest facts on and support for COVID-19 and vaccines, along with information on how we’re supporting our members and communities through the COVID-19 pandemic.
More COVID-19 vaccine stories from WellTuned
- My COVID-19 Vaccine Story: Jamie Pate
- My COVID-19 Vaccine Story: Dr. Bertram Prosser
- Andrea Willis: why I’m getting vaccinated as soon as I can
- COVID-19 vaccines: Q&A with four BlueCross medical directors
- Suzanne Corrington: COVID-19 side effects: what to expect + tips for care
- Suzanne Corrington: how effective are the COVID-19 vaccines + what does that mean?
- Chris Andershock: how immunity works + 4 ways to boost your immune system
Get more information about specific health terms, topics and conditions to better manage your health on bcbst.com. BlueCross BlueShield of Tennessee members can access wellness-related discounts on fitness products, gym memberships, healthy eating and more through Blue365®. BCBST members can also find tools and resources to help improve health and well-being by logging into BlueAccess and going to the Managing Your Health tab.